With increasing concerns regarding antimicrobial resistance in livestock, there is a widespread need for sustainable alternatives that can enhance health and productivity in poultry production. Ginger (Zingiber officinale Roscoe) is a phytobiotic known for its diverse health benefits, including growth promotion and improvement of intestinal function, and has therefore been evaluated for its effectiveness in the gastrointestinal tract of poultry. The present study investigated the effects of standardized ginger extract on intestinal morphology, microbiota composition, and growth performance in broiler chickens.
A total of 200 one-day-old broiler chicks (Ross 308) were randomly allocated to four dietary groups: a control group receiving a basal diet and three experimental groups receiving the basal diet supplemented with 2.5 g/kg, 5 g/kg, and 10 g/kg of ginger extract. Performance results demonstrated that dietary supplementation with ginger at a level of 5 g/kg significantly improved feed efficiency without exerting any negative effect on final body weight, while feed intake in broiler chickens was significantly reduced at higher doses of ginger extract. Broilers receiving 5 g/kg ginger exhibited a significantly higher villus height to crypt depth ratio in both the duodenum and jejunum.
In conclusion, supplementation of ginger extract at a level of 5 g/kg resulted in improved feed efficiency, intestinal morphology, and microbiota composition.

Nowadays, due to the increased use of antibiotics and the growing trend of antimicrobial resistance, there is a continuous emphasis on reducing antibiotic consumption. Consequently, there is a strong demand for sustainable alternatives that can maintain production efficiency while enhancing growth and health. Phytobiotics are plant-derived compounds that have emerged as promising candidates for this role. Unlike antibiotics, phytobiotics do not induce microbial resistance and do not leave harmful residues that may compromise animal health or consumer safety.

Among phytobiotics, ginger (Zingiber officinale Roscoe) has long been widely recognized for its medicinal properties in traditional medicine and is increasingly being studied for its applications in animal nutrition. Ginger rhizomes contain more than 400 compounds, with major constituents including carbohydrates (50–70%), lipids (3–8%), terpenes, and phenolic compounds such as gingerols, shogaols, gingerdione, and gingediol. These bioactive molecules play roles in various biological activities, including enhancement of digestive performance through stimulation of salivary and gastric secretions and increased activity of digestive enzymes, which collectively improve nutrient digestibility and feed efficiency.
In addition, ginger supplementation has been associated with improvements in intestinal morphology, enhanced nutrient absorption, and modulation of gut microbiota. Ginger exhibits antimicrobial effects against pathogenic organisms such as Escherichia coli, Salmonella spp., and Clostridium perfringens, thereby contributing to intestinal homeostasis and improved nutrient utilization. Ginger also demonstrates significant antioxidant and anti-inflammatory properties, mainly through upregulation of endogenous antioxidant enzymes and suppression of pro-inflammatory cytokines.
While most animal studies have utilized ginger root powder, it is hypothesized that ginger extract may provide greater efficacy and applicability. Therefore, the aim of the present study was to evaluate the effects of different concentrations of standardized ginger extract on growth performance, intestinal morphology, and microbial composition in broiler chickens.
Experimental Conditions
Two hundred one-day-old broiler chicks (Ross 308) with an average body weight of 44.5 g were obtained from a local hatchery and randomly assigned to four dietary treatment groups. Each group consisted of five replicates with ten birds per replicate. Birds were fed the following diets: broilers in the control group (C) received a basal diet, while broilers in the experimental groups received the basal diet supplemented with 2.5 g/kg standardized ginger extract (G1), 5 g/kg feed (G2), and 10 g/kg feed (G3). The experiment was conducted using a completely randomized design. Continuous lighting, ad libitum feeding, and unlimited access to water were provided. The ambient temperature was maintained at 32°C during the first seven days and then gradually reduced by 1°C every two days until reaching 24°C, which was maintained for the remainder of the experiment.
Diet Formulation
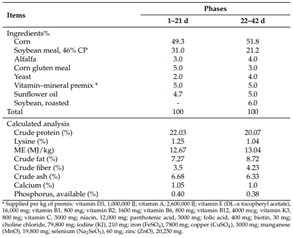
The diets were formulated according to specifications to meet the nutritional requirements of Ross 308 broiler chickens. All feed mixtures were prepared at once. The ginger extract was first mixed with a premix and then incorporated into the remaining feed ingredients. Subsequently, before feeding to the birds, the diets were stored in sealed containers. Broilers were fed a starter diet (days 1–21) and a grower diet (days 22–42). The formulation of the basal diet, along with the calculated chemical composition, is presented in Table 1.
Sample Collection
On day 42, two broilers from each cage (a total of 40 birds) were randomly selected and euthanized. Intestinal tissue samples (0.5 cm in length) were collected transversely from each intestinal segment for morphometric measurements. The intestinal segments were gently rinsed with phosphate-buffered saline to remove digesta and fixed in 10% formalin. Simultaneously, a 3-cm segment of the ileum containing digesta was collected.
Performance Analysis
Body weight (BW) of broilers was measured after 12 hours of feed withdrawal at the beginning of the experiment (day 1) and on days 14 and 42. Feed intake was recorded at the same time intervals. Feed conversion ratio (FCR) was calculated for the starter period (days 1–21), grower period (days 22–42), and the entire rearing period (days 1–42).
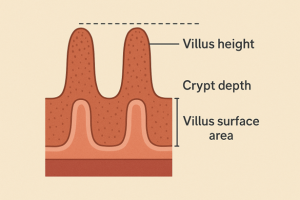
Intestinal Morphometry
Transverse sections with a thickness of 5 μm were prepared and stained. Histological preparations of intestinal tissue samples were examined using a light microscope. Analyses were conducted to measure villus height, crypt depth, and villus surface area. Data were obtained from the average of 15 villi per sample. Intestinal villi were measured at 10× magnification. Villus height was determined by measuring the distance from the tip of the villus to the junction between the villus and crypt.
Finally, the mean villus height of the intestine, calculated from eight measurements per bird, was averaged across ten birds to represent group mean values. The villus height to crypt depth ratio was calculated from the measured values
Statistical Analysis
Data related to growth performance, intestinal morphology, and microbiota composition and diversity were analyzed using statistical software (Statistic).
Performance Indices
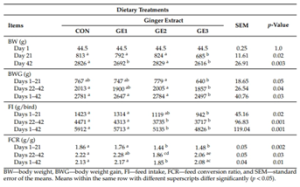
Growth performance indices of broilers fed ginger extract are summarized in Table 2. Significant differences in body weight among dietary treatments were observed on day 21 of the study. Broilers in the G2 group achieved the highest body weight compared with other groups. A significant difference in body weight was observed between all groups and the G3 group on day 21. On day 42, the highest body weight was recorded in the C and G2 groups compared with the G1 and G3 groups. Between days 1 and 21, a significant increase in body weight gain was observed in the G2 group compared with the G3 group.
During the period from day 22 to day 42, higher weight gain was observed in both the C and G2 groups compared with the G3 group. Overall, body weight gain in the C, G1, and G2 groups was significantly greater than in the G3 group. Higher feed intake was recorded in the C and G1 groups compared with the G3 group during the first three weeks of the experiment. Between days 22 and 42, feed intake was higher in the C and G1 groups compared with the G2 and G3 groups. Over the entire experimental period (days 1–42), the highest feed intake was recorded in the control group, such that the C and G1 groups showed significantly higher feed intake than the G2 and G3 groups. Feed intake exhibited an approximately linear decrease with increasing ginger extract concentration throughout the experiment. Over the entire experimental period, the lowest feed conversion ratio was observed in broilers from the G2 group compared with other groups.
Quantitative Measurements of Intestinal Tissue
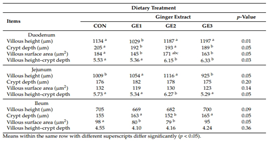
Feeding broiler chickens with ginger extract at different concentrations affected the morphometric parameters of the small intestine, as shown in Table 3. On day 42 of the experiment, duodenal villi were significantly shorter in the G1 group compared with the other groups. The greatest crypt depth was recorded in the control group compared with the G1 and G3 groups. The villus height to crypt depth ratio was significantly higher in the G2 and G3 groups than in the control and G1 groups.
In the jejunum, significantly longer villi were observed in the G2 group compared with the CN and G3 groups. No statistically significant differences were observed among the groups in terms of crypt depth or villus surface area. The villus height to crypt depth ratio in the G2 group was significantly higher compared with the other groups.
In the ileum, crypt height was significantly greater in the G1 and G3 groups compared with the G2 group. The villus surface area of the jejunum was significantly larger in the control group compared with the G1 and G2 groups.
Microbiome Composition and Diversity
The composition of the intestinal microbiota is presented in Figure 1A. The dominant phyla, in descending order, were Firmicutes, Proteobacteria, Actinobacteria, Campylobacterota, and Bacteroidetes, accounting for 62.4%, 22.9%, 2.65%, 2.03%, and 1.43% of the total sequences, respectively.
In the G2 and G3 groups, a significant decrease in the relative abundance of Proteobacteria and a significant increase in the proportion of Firmicutes were recorded compared with the control group. In addition, a significant reduction in the relative abundance of Campylobacterota and Bacteroidota was observed in these groups. More than 30 genera were identified in all samples; however, only the 10 most abundant genera are displayed in the histogram in Figure 1B.
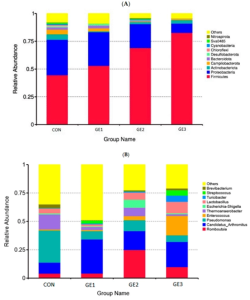
In the control group, the dominant bacterial communities consisted of the genera Pseudomonas, Thermoanaerobacterium, Candidatus Arthromitus, and Lactobacillus. In the ginger extract–supplemented groups, a significant increase in the relative abundance of the genera Candidatus Arthromitus and Romboutsia, along with a significant decrease in Pseudomonas and Thermoanaerobacterium, was observed.
Furthermore, in the G2 group, the abundance of bacteria belonging to the Escherichia–Shigella genus was significantly higher compared with the other groups. In the G3 group, the abundance of bacteria belonging to the genera Enterococcus and Lactobacillus was significantly higher than in the other experimental groups.
Production Performance
Growth performance in broiler production is a critical parameter for evaluating efficiency and economic profitability, as it reflects the effects of numerous internal and external factors, including genetics, nutritional strategies, environmental conditions, health status, and farm management practices. In the present study, the effects of ginger extract supplementation at different concentrations on performance parameters were evaluated.
Although body weight on day 1 was uniform among the groups, by day 21, body weight in the G3 group was significantly lower. This trend continued until day 42, resulting in the lowest final body weight among all treatment groups. These findings are consistent with previous reports indicating a negative effect of ginger on weight gain at levels ranging from 6 to 60 g/kg.
When body weight was analyzed in relation to feed intake, a significant reduction in feed consumption was observed in the G3 group up to day 21 and continued through the second feeding phase, suggesting that the lower final body weight may be attributed to reduced feed intake.
Explaining the observed condition in the G1 group is somewhat more complex. Nevertheless, it was found that this group, which received the lowest dose of the tested additive, exhibited a markedly lower final body weight. No difference in body weight was observed on day 21; however, during the second fattening phase, weight gain decreased compared with the control and G2 groups, despite unchanged feed intake.
Conversely, the final body weight of the G2 group was comparable to that of the control group, despite significantly lower feed intake during both fattening phases. These results are consistent with findings reported by several authors, in which no correlation between ginger supplementation and body weight gain in broiler chickens was observed.
However, some studies have reported positive effects of ginger supplementation on broiler body weight. According to these reports, gingerol, one of the active components of ginger, stimulates the secretion of digestive enzymes, thereby improving digestion and increasing the utilization of ingested nutrients. The pancreas is stimulated to produce greater quantities of digestive enzymes, resulting in enhanced digestion and nutrient absorption, which supports higher growth rates.
Feed Intake and Feed Conversion
Regarding feed intake in broiler chickens, a significant adverse effect on consumption, particularly with increasing dosage, was immediately observed following supplementation with the tested product. Lower feed intake was recorded in all experimental groups compared with the control group.
One possible explanation for this may be attributed to the reduced villus height to crypt depth ratio observed in all intestinal segments of the G1 group, leading to decreased nutrient absorption. The negative effect of high ginger doses on feed intake was also reported by Herawati et al., who observed a significant reduction in feed intake at a dose of 20 g/kg, although no adverse effect on final body weight was detected.
Feed intake is one of the key parameters that best describes livestock production efficiency, as feed represents the largest cost for producers. Production costs can be significantly reduced by improving feed utilization and by adding substances that enhance digestibility and absorption, particularly during periods of significant raw material price fluctuations.
In the present study, a significant improvement in feed conversion ratio was observed in broilers of the G2 group compared with the other groups. This finding is consistent with results reported by several authors, in which a lower feed conversion ratio was also observed in broilers receiving similar doses of ginger (5 g/kg).
It is assumed that the improvement in feed conversion ratio is related to the stimulation of gastric secretions and salivary gland activity. This stimulation is believed to result in a reduction of pathogenic microorganisms and an overall decrease in microbial fermentation in the intestine, thereby enhancing digestive and absorptive capacity. In contrast, a negative effect on feed intake was observed only when supplementation was administered at very high concentrations (30 g/kg). Conversely, no significant effect of ginger supplementation on feed intake was reported in other studie
Intestinal Morphometry
To explain the effects of ginger supplementation on the morphometric parameters of the chicken small intestine, it must be considered that the chemical composition of the diet is a key factor influencing intestinal structure and, consequently, its absorptive capacity. This, in turn, ultimately affects broiler growth performance.
Intestinal villi rapidly adapt to changes in the luminal environment, which is largely shaped by dietary composition. Accordingly, increased villus height is associated with an increased absorptive surface area and enhanced nutrient absorption capacity. The crypt region is responsible for the production of enterocytes that migrate from the base to the tip of the villi, replacing senescent cells that are shed. In the lumen, these epithelial cells are continuously and rapidly renewed, a process that is essential for maintaining normal intestinal function.
Villus height and the villus height to crypt depth ratio are widely used as positive indicators of intestinal mucosal health, whereas crypt depth alone is often considered a negative indicator of intestinal health. Longer villi are associated with greater activity of mucosal digestive enzymes, and a higher ratio is generally associated with improved nutrient absorption. In contrast, deeper crypts indicate poorer absorptive function, as they reflect increased epithelial turnover. Deeper crypts may also indicate more rapid regeneration of the intestinal lining.
Furthermore, shorter villi accompanied by deeper crypts have been associated with a reduced number of absorptive cells and an increased proportion of secretory cells, leading to increased mucin secretion. Changes in the quantity or composition of mucin at the intestinal mucosal surface can impair nutrient absorption and may increase the energy required to maintain intestinal function.
In the present study, the effects of ginger supplementation on histological parameters were observed in different segments of the small intestine. In the duodenum, a significant reduction in villus height in the G1 group compared with the control group was recorded, accompanied by reduced crypt depth. A significant reduction in villus surface area was also observed in all treated groups compared with the control group. However, the villus height to crypt depth ratio was significantly higher in the G2 and G3 groups compared with the control group.
In the jejunum, villus height was significantly increased in the G2 group, while crypt depth remained unchanged compared with the control group. In the ileum, no significant differences in villus height were observed among the groups; however, reduced villus surface area was recorded in the G1 and G2 groups.
In the present study, positive morphological changes were observed in the duodenum and jejunum of the G2 group. Specifically, duodenal villi were elongated with a significant reduction in crypt depth, while the jejunum exhibited increased villus height and a higher villus height to crypt depth ratio.
In contrast, other studies have reported improved performance in broiler chickens without significant changes in villus height or surface area. However, an increase in the surface area of duodenal epithelial cells and enhanced mitotic activity in crypts has been attributed to ginger supplementation.
Microbiome
The gut microbiome, which comprises billions of microorganisms, is recognized as a complex ecosystem that mediates interactions between the host and its environment. The intestinal microbiome plays a critical role in maintaining animal health and is closely associated with digestion, absorption, metabolism, immunity, and disease susceptibility.
In addition, the microbiome influences carbohydrate fermentation, particularly polysaccharides, thereby enhancing nutrient absorption and energy supply in the animal organism. Although the therapeutic use of antibiotics is effective in combating bacterial infections, it can lead to dysbiosis of the intestinal microbiome. This may result in the depletion of beneficial gut bacteria and the induction of inflammatory responses, leading to elevated levels of pro-inflammatory cytokines.
Since phytobiotics generally do not induce bacterial resistance or inflammatory responses, their use is considered essential for maintaining the structural stability and microbial diversity of the intestinal microbiota.
Previous research has demonstrated that intestinal health benefits from high bacterial population diversity, as competition for resources and colonization among microbial communities reduces the likelihood of pathogen proliferation and subsequent infection. In the present study, no statistically significant differences in bacterial genus diversity or richness were observed in the ileum of chickens fed ginger extract. This stability in microbiome diversity suggests that ginger extract does not disrupt microbial balance.
Some authors believe that gut microbiota dynamics change in response to dietary modifications and age, whereas others argue that the microbiome is more strongly influenced by age than by diet.
In broiler gut microbiota analysis, an increase in the relative abundance of Firmicutes was observed, showing a positive correlation with the administered ginger dose, while the proportion of Proteobacteria simultaneously decreased. According to previous studies, Firmicutes have been identified as key contributors to butyric acid production, which serves as an energy source for the growth and maintenance of intestinal epithelial cells.
It has been shown that the Firmicutes-to-Bacteroidetes ratio plays a fundamental role in nutrient absorption and intestinal homeostasis in poultry. A higher proportion of Firmicutes has been associated with suppression of pathogenic bacteria, restoration of intestinal homeostasis, and improved nutrient absorption.
Conversely, an increased abundance of Bacteroidetes has been associated with impaired nutrient absorption and disrupted intestinal microbial function. Previous research has demonstrated that a high relative abundance of Proteobacteria is an indicator of intestinal imbalance, with elevated levels reflecting poor growth or unstable microbiota composition. In addition, Proteobacteria include several pathogens common to both humans and animals, such as Escherichia, Salmonella, and Campylobacter, along with other clinically important genera.
In the present study, supplementation with ginger extract significantly reduced the relative abundance of harmful Proteobacteria in the G2 and G3 groups, thereby contributing to the maintenance of a more balanced intestinal microbiota.
These results are consistent with those reported by authors who observed an increase in this bacterial genus in broiler chickens fed Bacillus subtilis probiotics. In all broiler groups receiving the tested product, a significant reduction in Pseudomonas bacteria was observed compared with the control group. Previous studies have reported increased proportions of Pseudomonas bacteria in the intestines of immunocompromised animals due to bacterial infection.
In the G2 group, an increased proportion of Escherichia–Shigella bacteria was observed. This finding is somewhat surprising, as this group achieved the best production results and no mortality was recorded during the experiment. Elevated levels of bacteria belonging to this genus can lead to colibacillosis in poultry and have been observed in the microbiome of broilers experimentally infected with Escherichia coli.
A significantly higher proportion of Lactobacillus bacteria was found in the G3 group compared with the other groups. This finding is consistent with reports from authors who tested ginger products in broiler chickens. Increased proportions of Lactobacillus are considered desirable due to their positive effects on the intestinal immune system and their stimulation of lactate-utilizing, butyrate-producing bacteria.
Additionally, a significantly higher proportion of Enterococcus bacteria belonging to probiotic species—such as Lactobacillus, Bacillus, Bifidobacterium, Streptococcus, and Faecalibacterium—was observed in the same group, exerting a positive effect on broiler health and performance.
Conclusion
It was determined that the addition of ginger extract at a concentration of 5 g/kg improved feed efficiency and small intestinal morphometric characteristics. Importantly, the evaluated additive did not disrupt microbial homeostasis and was shown to significantly support the maintenance of a favorable intestinal microbiota. Based on these findings, this inclusion level can be considered effective and potentially beneficial in broiler chicken nutrition.

